iUTAH Team - Undergraduate iFellows

Sandra Udy (Young)
Utah State University
Mentors:
Faculty: Michelle Baker, USU
Graduate: Beth Ogata, USU
Research Focus:
Research Focus Area 1
Major:
Environmental Soil and Water Science
Biography:
Sandra Udy (Young) is an Environmental Soil and Water Science major graduating December of 2016. Sandra enjoys spending time with her husband and spending time outdoors. She especially enjoys gardening. She loves studying soil and has participated in research dealing with soil contaminants and soil reclamation. Field work is her favorite part of her research experience. She is excited to have more research experience focusing on water dynamics this summer. After graduation she plans to pursue a career in soil testing and analysis.
iFellow Presentation:
Microbes vs Nutrients: Understanding Nutrient Pollution in Streams

Presented by: Sandra Udy (Young)
July 2016
Weekly Recap:
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11
Week 1: May 16-20, 2016
I spent some time with my peer mentor, Elizabeth Ogata, going over how to prepare the agar substrates for the field. She showed me around the lab and introduced me to various parts of the research that I will be participating in. I am excited that I will have the opportunity to expand on the research done last summer on pharmaceuticals and their effects on the biofilm in the rivers in Utah. I haven't done a lot with water and nutrient relationships, so I am excited to learn about the effects that nutrient runoff has on river ecosystems.
Week 2: May 23-27, 2016
This week I began working on preparing agar cups to be brought out to the field next week. This involved heating up water and adding the agar. The solution was autoclaved and a nutrient amendment was added. This was separated into little cups and glass discs were placed on top. Once these were cooled I siliconed these to an L bar and zip-tied them down. This went way better than I was expecting, especially since my mentor was out of town for a conference. I did learn that zip-ties are harder to use than I remembered. I also realized that the autoclave is a super cool machine. I am excited to go out to the field next week.

This torpedo-looking anvil was the tool we lowered into the Logan River in order to measure the velocity of the water at certain depths.
Week 3: May30-June 6, 2016
On Tuesday of this week I completed assignment 1 for the research integrity course. Then on Wednesday we were able to go out to the field to deploy the L-bars into the river. I drove down with Elizabeth Ogata, my peer mentor, to the upper Provo River just below Jordanelle Reservoir. Here we zip-tied the L-bars to bricks. These were then deployed in the center of the river. We collected dissolved oxygen and light levels at the surface and river floor. It was exciting to learn more about how the microbes will begin to grow on the glass discs and how the nutrients will slowly diffuse through the agar and be used by the microbes. These L-bars will be collected in approximately two weeks and analyzed to determine what nutrients are being used by the microbes. Thursday and Friday were spent in the lab running tests on previously prepared hemi-cellulose mixtures. I am excited to continue learning and hope I can apply all that I have learned so far.

Zip-tying the L-bars with the agar gel cups onto the bricks before deploying them in the river.
Week 4: June 6-10, 2016
This week I have had the opportunity to learn about the DNA-SIP process. SIP stands for stable isotope probing. It uses an isotope of an element like nitrogen or carbon. This nutrient is added with bacteria, for example, and the bacteria digest the nutrient. Because the isotope is different than what would normally be taken up, the nutrient can be tracked in the bacterial DNA. This is done by collecting the bacterial DNA. This week I used a DNA processing kit to prepare the DNA for analysis. This will be put into a centrifuge. The centrifuge will separate DNA with the isotope (because it is a different density). The DNA that has the isotope in it can then be compared with DNA from a bacterial DNA database. This way I can determine which types of DNA are using the nutrient.

Week 5: June 13-17, 2016
This week began with a cohort session. This cohort session was a wonderful chance to get some hands on experience in presenting and in the field. I was extremely grateful for the presenting experience. Presenting a poster is very different than presenting a powerpoint. I liked that it gave me a chance to bounce ideas off of other iFellows and see how they outlined their poster so far. It also was a great experience to learn how to share what I learned to different audiences. I liked how we talked about getting to know the audience before we began to share with them. I also had the chance this week to start to learn about R. I worked with Beth Ogata to figure out what different lines of code mean for the output of graphs and charts. I still have a lot to learn but it was pretty exciting when I was able to produce a chart! Next week begins with fieldwork and collecting of the NDS cups we deployed earlier. I am excited to tie in all of the things I learned.
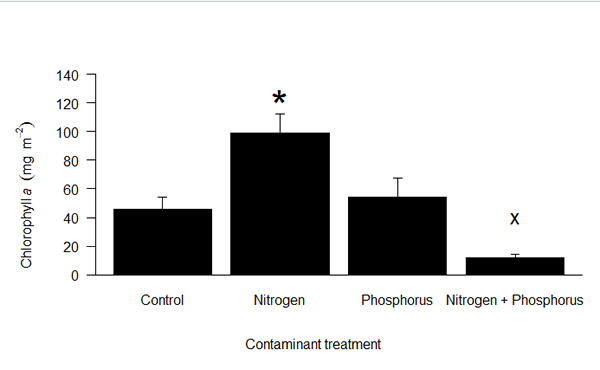
Week 6: June 20-24, 2016
This week was very busy! Monday Beth and I collected our NDS samples from the Provo River. These were brought back to the lab and separated into their different treatment types. Nutrient Limiting samples were put into cups to be analyzed for ash free dry mass. These NDS samples will also be analyzed to determine the amount of chlorophyll a on them from the biomass that grew. 15N samples were frozen to be taken to BYU for DNA-SIP analysis. Hemicellulose samples were placed in mason jars to be incubated for a week. These will be incubated with treatments of nitrogen, phosphorus, and a combination of nitrogen and phosphorus. The day 7 incubation will be compared with the day 0 incubation to see if the treatments of nitrogen and/or phosphorus impacted uptake of the hemicellulose. I started to learn how to use the Aqualog this week and in later weeks I will use it to analyze samples from the hemicellulose experiment. It has been exciting to see how the nutrients affect the biofilm growth. It will be really interesting to see which nutrients affect the biofilm growth the most. Lots of exciting things to learn!



Week 7: June 27-July 1, 2016
This week began with another iFellow session discussing presenting posters, attending conferences, and networking. I really liked getting a new perspective on networking and all of the people that provide a strong base for continuing research. We also had the opportunity to learn more about the GAMUT system and went to a site. I really liked learning about the YSI probe and how it is being used to record new information. My week continued with a trip down to BYU to process more DNA for the SIP process. We had a lot more success this week and learned some more techniques to make the process better. The centrifuge was struggling, so hopefully with that working again we will be able to complete the DNA-SIP process and discover how the bacteria used the nitrogen. I also used the aqualog machine this week. It helps to characterize organic matter in our samples. It was cool to see how different nutrient amendments influenced the readings for the aqualog. I am excited to find out what the readings mean and what types of protien are in each sample. Overall it has been an exciting week and I'm excited to send in the rough draft for my paper and prepare my poster for next week!
Week 8: July 4-8, 2016
This week started out with a bang! Well, with July fourth that is. On Tuesday Beth Ogata and I went down to BYU to extract more DNA. This time extracting went well and we were able to extract twenty four of the discs from our NDS cups. I am excited to have the ultracentrifuge working so that we can see the DNA-SIP process from beginning to end. The discs that had the glycine amendment didn't seem to have as much growth on them, but that could be because the autotrophic algae don't like the organic form of nitrogen as much. I am excited to see what happens and what types bacteria prefer what form of the nitrogen. I also got to work with R Studio this week some more and learned some simple troubleshooting tips that were super helpful. This week ended with presenting our posters at an ifellow cohort session. This was a wonderful opportunity and I really enjoyed seeing new ideas of ways to present and how different poster styles were organized. I gained some new insight on how to improve my poster.
Week 9: July 11-15, 2016
This week began with another round of DNA extracting at BYU. It has been getting a lot easier and I am always excited to see how the DNA purification process works. I am excited to continue the stable isotope probing process to the next step. This week I also had the opportunity to work on the short scientific film for the project. It was a lot more nerve-racking than I thought it was going to be but I'm excited to have the finished product. I have really enjoyed how this experience has introduced me to many different parts of research that I have never had the opportunity to participate in before. I am really grateful that I had the chance to present my research poster at the end of this past week. I have attended poster sessions before but I've never had a chance to be part of the poster making progress or the presenting process. It was challenging in ways I wasn't expecting and I am very grateful for the opportunity. It required me to have a much deeper understanding about every part of the research I am participating in and is a perfect introduction into what graduate school will be like.
Week 10: July 18-22, 2016
Sandra Udy explains how to create a nutrient diffusing substrate.
All content provided on this iUTAH Team - Undergraduate iFellows weekly recap is unedited, updated by each participant to provide a review of their progress, and is for informational purposes only.


